生殖功能的調節主要由糖蛋白激素,卵泡刺激素(FSH)、促甲狀腺激素(TSH)、黃體生成素(LH)和絨毛膜促性腺激素(hCG)進行。糖蛋白激素包含α 亞基 & β 亞基
hCG、FSH 和 LH 有相同的α 亞基,包含 92 個氨基酸,
hCG、FSH 和 LH 之 β 亞基對於所有三種激素是特異性和獨特的,可引發不同的生物學和免疫學特性。
聚醣glycans在促性腺激素與其各自受體的結合中發揮著重要作用,識別相關聚醣及其藥理學特性非常重要。
蛋白質糖基化Protein glycosylation 是一種複雜的翻譯後修飾post-translational modification (PTM),涉及glycans在特定位點的附著,最常見的是天冬酰胺(N 連接)或絲氨酸/蘇氨酸(O 連接)殘基。糖基化glycosylation模式在蛋白質的溶解度、穩定性和生物活性中起著至關重要的作用。
新的聚醣 glycan是一種四觸角物種tetra antennary , FSH 與受體的結合中發揮重要作用,具有更高的生物活性、更低的清除率和更長的半衰期。
由於糖基化的差異,糖蛋白的生物活性和半衰期可能不同。
質譜儀mass spectrometry MS技術的進步,可以鑑定微量的相關聚醣。MS 可進行定量和定性分析,例如分子量測定、結構闡明或序列測定。
https://www.sciencedirect.com/science/article/abs/pii/S1642431X18300949
https://link.springer.com/article/10.1007/s00216-011-4923-5
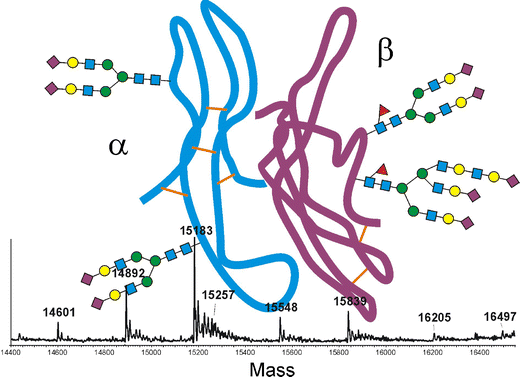
Glycan mapping of recombinant human follicle stimulating hormone by mass spectrometry
Analysis of recombinant human follicle-stimulating hormone (FSH) by mass spectrometric approaches
- Thorough analysis of the heterodimeric heavily glycosylated protein of rFSH is a prerequisite for the evaluation of production batches as well as for the determination of “essential similarity” of new biosimilars.
- The concerted application of different liquid chromatography-mass spectrometry methods enabled the complete depiction of the primary structure of this pituitary hormone.
- Sequence coverage of 100% for the α- as well as the β-chain was achieved with tryptic peptides.
- Most of these peptides could be verified by tandem mass spectrometry. Site-specific analysis of all four glycosylation sites was, however, not possible with tryptic but with chymotryptic peptides. Quantification of the glycoforms of each glycopeptide was accomplished with the software MassMap®. Both protein subunits gave interpretable mass spectra upon S-alkylation and separation on a C5 reversed-phase column. Glycan isomer patterns were depicted by separation on porous graphitic carbon, using mass spectrometric detection for the evaluation of the glycopeptide liquid chromatography-electrospray ionization data. The currently marketed product Gonal-f™ and a potential biosimilar were compared with the help of these procedures.


